Pharmaceuticals and biotechnology industry
Improving your production quality
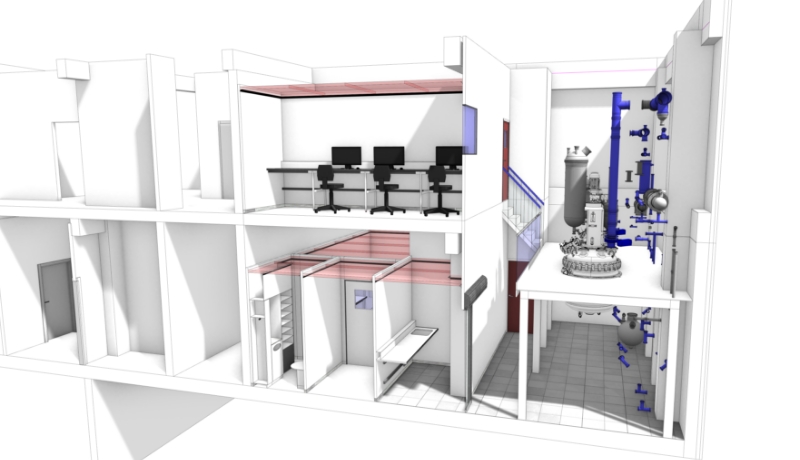
Product manufacturing in the pharmaceuticals and biotechnology industry requires contamination control within the production environments. Creating a compliant product is dependent on both the integrity of the materials that make up the medicine and its manufacturing and packaging in either aseptic conditions or a controlled environment.
These two conditions require full contamination control of premises and equipment, depending on the production phase.

Cleanrooms (ISO 14644-1) are therefore designed to control particle concentration in order to minimise particle introduction, generation and retention inside rooms. The rooms must be easily decontaminable and therefore resistant to the most common cleaning agents (N2O2, NaOH). Conditions such as temperature, humidity and relative pressure are also maintained at a precise level (defined in line with ISO 14644-1). Pressure cascade systems maintain positive or negative air pressure between classified areas. These rooms are monitored by sensors integrated into the rooms alongside electricity, network and air handling systems.
Numerous factors have a direct influence on compliant room operation, including the design, materials, filtration techniques (AHU, HVAC), type of activity, and number of people in the room.
LSB brings together the best materials, techniques and professional skills to create rooms that serve as functional equipment.
These rooms meet all requirements, from design to installation, materials selection and technical management.
Our expertise and equipment will help you reach cleanroom GMP (Good Manufacturing Practices) level for your activity and process.